Thank you to all that voted during this years CommunityVotes Windsor campaign. We are pleased to announce that we have won the 2023 PLATINUM AWARD for being VOTED BEST IN OSTEOPATHS. We truly appreciate the ongoing support!
COMMUNITYVOTES WINDSOR 2023 PLATINUM WINNER
VOTED BEST IN OSTEOPATHS


NEWS
Please note that all our practitioners book one-on-one appointments to help you reach your health goals. Failure to comply with this policy, sacrifices a time that could be given to another patient in need. We understand that some things are out of your control, such as unexpected traffic conditions, construction, accidents, etc. If this does occur to you and it is safe for you to do so, please call us to inform us. It is best practice to do your best to arrive 5 minutes prior to your appointment, so that we can get you settled into your exam room and ready for the practitioner. This will aid our office in being punctual. Our office would love nothing more than to never be put into the situation we must enforce this policy. We hope you understand that this policy only benefits you as a patient, allows our clinic to be fair and respectful to all our patients.
EFFECTIVE JULY 10, 2023, the following changes will be made to the “LATE CANCELLATION AND NO-SHOW POLICY”:
LATE CANCELATIONS:
NO SHOWS:
SHOWING UP LATE TO YOUR SCHEDULED APPOINTMENT:
MASSAGE THERAPY ADDITIONAL:
Spencer Jean, DO & Associates is pleased to welcome Jordyn Klein, RMT to our clinical team as our Registered Massage Therapist. Jordyn is gradually coming back from maternity leave and is currently accepting new clients/ patients. Patients can schedule their own appointments through our secured online scheduling and electronic medical records system or by calling our clinic at 519-900-5131.
Jordyn is a Registered Massage Therapist with the College of Massage Therapists of Ontario (CMTO). She attended the Canadian College of Health Science & Technology in pursuit of her Diploma in Massage Therapy and Hydrotherapy – ultimately, graduating with honours and later serving as an instructor’s assistant for the program while writing her board examinations. She is well versed in the therapeutic applications of massage therapy for the treatment of musculoskeletal conditions as well as general wellness and relaxation. Outside of the clinic, Jordyn enjoys spending time with her son Theodore and her husband Spencer.
You may have met Jordyn while she was working reception while awaiting the birth of her son Theodore. What you may not know, is that Jordyn is Spencer’s wife.
I couldn’t be happier to reintroduce Jordyn to our clinic – this time as our Registered Massage Therapist. Although being Jordyn’s husband may make me slightly biased, I can say that I completely trust my own physical health with her therapy. Watching her complete her studies has brought a new light towards the profession of Massage Therapy. The educational material is quite in-depth and when applied correctly it can make a profound positive impact on the patient’s health. Having Jordyn as part of my patient’s healthcare team, will significantly benefit their treatment plans and above all else – their quality of life. Please join me in welcoming Jordyn to our clinical team. – Spencer Jean, DO, DO(MP), MBA
MEET JORDYN KLEIN, RMT
SCHEDULE AN APPOINTMENT WITH JORDYN
REGISTERED MASSAGE THERAPY APPOINTMENTS ARE COVERED BY MOST EXTENDED INSURANCE PLANS.
Spencer Jean, DO & Associates is pleased to welcome Noura Jabbour, RD, MSc. to our clinical team as our expert Registered Dietitian. Noura will be accepting New Patients starting Saturday, February 25, 2023 for nutrition assessments. Patients can schedule their own appointments through our secured online scheduling and electronic medical records system or by calling our clinic at 519-900-5131. Finding the right Dietitian to join our team was a priority for our clinic to support our patient’s treatment plans, overall health and optimizing their quality of life.
Noura is a licensed Registered Dietitian in Ontario and has been practicing since 2018. She has experience in clinical dietetics where she provides credible, evidence-based nutrition information that is tailored to each client’s specific needs. Noura is currently employed at Windsor Regional Hospital where she provides nutrition care for different types of conditions, including cancer, weight gain, weight loss, cardiovascular disease, type 2 diabetes education (including gestational diabetes), and gastrointestinal diseases, to name a few. She has also worked as an independent contractor for Long-Term Care, as a Registered Dietitian for the Windsor-Essex County Health Unit, and as Dietitian Project Specialist where she helped to oversee the food service management of acute care facilities across Canada. With professional experience in clinical nutrition, public health nutrition and food service nutrition, she comes equipped with a well-rounded background to help her client’s understand how best to make lifestyle changes in a way that they can understand and implement for long term success.
Education:
Specialties:
Optimizing dietary intake effects chronic pain: Sub-optimal dietary intake can be the result of a number of factors which need to be addressed:
Injury recovery can be influenced by nutrition interventions.
MEET NOURA JABBOUR, RD, MSc.
Nutrition interventions have a significant effect on pain reduction.
DIETITIAN SERVICES ARE COVERED BY MOST EXTENDED INSURANCE PLANS.
The clinic will be closed from October 26 to November 6 as Spencer & Jordyn are expecting the birth of their first born during these dates.
The clinic will resume regular hours starting November 7.
If you have active treatment plans, please contact us to schedule your appointments before and after these dates. If you are concerned about not being able to have a treatment during these dates, please let Spencer know and he will be happy to guide you in the right direction so you do not fall behind on your progress.
Thank you for your continued support and understanding.
Just a reminder… you can schedule your appointments online through our online booking portal at https://spencerjeando-and-associates.janeapp.com/
We are grateful to announce that we have won the COMMUNITYVOTES WINDSOR 2022 “PLATINUM” Award in the category of “Healthcare Osteopaths”. This means that our Manual Osteopath, Spencer Jean, DO, DO(MP), MBA, has been rated once again as the Best Manual Osteopath in Windsor-Essex County by our community.
We’d like to thank our 4000+ patients that have been under Spencer’s care since 2015. Without you and your support this wouldn’t be possible. It is a great honour to have your trust of healthcare.
Thank you once again for your continued support.
Patients,
Ontarians should continue to wear a mask if they feel it is right for them, or are at a high risk for severe illness, recovering from COVID-19, or have symptoms of the virus or are a close contact of someone with COVID-19.
Masking is no longer a requirement of our associations and colleges. If you wish to continue to wear a mask to your appointment, you are welcome to do so. If you would like your practitioner to wear a mask during your appointment, please let us know and we are happy to accommodate.
Thank you.
You may have had TENs as a form of treatment in your past, heard of the treatment or even have a TENs machine at home to use for your condition. BUT, do you know what a TENs Machine actually does? How it works? Are you getting the most benefits out of the treatment? In this article, I’ll explain everything you as a patient need to know about TENs Treatment so you are confident in knowing how to properly use the machine at home (if you have one) or you are educated on how it can help you and your condition clinically.
TENS as a treatment technique is non invasive and has few side effects when compared with drug therapy. ***A TENS MACHINES PRIMARY GOAL IS PAIN RELIEF***
FREQUENCY (B): The machine will deliver discrete ‘pulses’ of electrical energy, and the rate of delivery of these pulses (the PULSE RATE or FREQUENCY (B) will normally be variable from about 1 or 2 pulses per second (pps) up to 200 or 250 pps (sometimes the term Hertz or Hz is used here). To be clinically effective, it is suggested that the TENS machine should cover a range from about 2 – 150 pps (or Hz). DURATION (C): In addition to the stimulation rate, the DURATION (OR WIDTH) OF EACH PULSE (C) may be varied from about 40 to 250 micro seconds. (a micro second is a millionth of a second). Recent evidence would suggest that this is possibly a less important control that the intensity or the frequency and the most effective setting in the clinical environment is probably around 200’s. The reason that such short duration pulses can be used to achieve these effects is that the targets are the sensory nerves which tend to have relatively low thresholds ( i.e. they are quite easy to excite) and that they will respond to a rapid change of electrical state. There is generally no need to apply a prolonged pulse in order to force a sensory nerve to depolarise, therefore stimulation for less than a millisecond is sufficient. BURST MODE (D): In addition, most modern machines will offer a BURST MODE (D) in which the pulses will be allowed out in bursts or ‘trains’, usually at a rate of 2 – 3 bursts per second. MODULATION MODE (E): may be available which employs a method of making the pulse output less regular and therefore minimizing the accommodation effects which are often encountered with this type of stimulation. Both the burst and modulation modes will be discussed in more detail in the following sections.
MECHANISM OF ACTION The type of stimulation delivered by the TENS unit aims to excite (stimulate) the sensory nerves, and by so doing, activate specific natural pain relief mechanisms. For convenience, if one considers that there are two primary pain relief mechanisms which can be activated : The Pain Gate Mechanism and the Endogenous Opioid System, the variation in stimulation parameters used to activate these two systems will be briefly considered. Pain relief by means of the pain gate mechanism involves activation (excitation) of the A beta (A?) sensory fibres, and by doing so, reduces the transmission of the noxious stimulus from the ‘c’ fibres, through the spinal cord and hence on to the higher centres. The A? fibres appear to appreciate being stimulated at a relatively high rate (in the order of 80 – 130 Hz or pps). An alternative approach is to stimulate the A delta (A?) fibres which respond preferentially to a much lower rate of stimulation (in the order of 2 – 5 Hz, though some authors consider a wider range of 2 – 10Hz), which will activate the opioid mechanisms, and provide pain relief by causing the release of an endogenous opiate (encephalin) in the spinal cord which will reduce the activation of the noxious sensory pathways. A third possibility is to stimulate both nerve types at the same time by employing a burst mode stimulation. In this instance, the higher frequency stimulation output (typically at about 100Hz) is interrupted (or burst) at the rate of about 2 – 3 bursts per second. When the machine is ‘on’, it will deliver pulses at the 100Hz rate, thereby activating the A? fibres and the pain gate mechanism, For some patients this is by far the most effective approach to pain relief, though as a sensation, numerous patients find it less acceptable than some other forms of TENS as there is more of a ‘grabbing’, ‘clawing’ type sensation and usually more by way of muscle twitching than with the high or low frequency modes.
Usually uses stimulation at a relatively high frequency (80 – 130Hz) and employ a relatively narrow (short duration) pulses though as mentioned above, there is less support for manipulation of the pulse width in the current research literature. Most patients seem to find best effect at around 200s. The stimulation is delivered at ‘normal’ intensity – definitely there but not uncomfortable. 30 minutes is probably the minimal effective time, but it can be delivered for as long as needed. The main pain relief is achieved during the stimulation, with a limited ‘carry over’ effect – i.e. pain relief after the machine has been switched off. FREQUENCY SELECTION : with all of the above mode guides, it is probably inappropriate to identify very specific frequencies that need to be applied to achieve a particular effect. If there was a single frequency that worked for everybody, it would be much easier, but the research does not support this concept. Patients (or the therapist) need to identify the most effective frequency for their pain, and manipulation of the stimulation frequency dial or button is the best way to achieve this. STIMULATION INTENSITY : As identified above, it is not possible to describe treatment current strength in terms of how many microamps. The most effective intensity management appears to be related to what the patient feels during the stimulation, and this may vary from session to session. As a general guide, it appears to be effective to go for a ‘definitely there but not painful’ level for the normal (high) TENS. There is a growing body of evidence that suggests that a ‘strong’ sensation, whichever mode is being used, might achieve better clinical effects. ELECTRODE PLACEMENT : In order to get the maximal benefit from the modality, target the stimulus at the appropriate spinal cord level (appropriate to the pain). Placing the electrodes either side of the lesion – or pain areas, is the most common mechanism employed to achieve this. There are many alternatives that have been researched and found to be effective – most of which are based on the appropriate nerve root level :
TENs is a great modality to incorporate to achieve pain relief for both acute and chronic conditions – When used appropriately. Although the primary goal of the treatment itself is not to cure or fix the condition, it’s mechanism of action to achieve pain relief will indirectly help you manage your condition or aid in the correction causing the painful stimuli. When there is less pain – you will be able to do more of your exercises and enjoy your daily routine better. For chronic pain, there is a strong correlation in how the brain perceives the painful area. A lot of the time, the brain believes there is still an issue going on in the area and sends a painful response – even if the initial injury has resolved. Using a TENs at home is a great way to help retrain the psychosomatic system of the brain to help it shut off the painful sensory receptors.
If you are unsure if your TENs Machine is set up appropriately for your condition to achieve the maximum benefit, please let us know and we would be glad to set it up correctly for you. If you do not have a TENs Machine for home use and you believe it may help you and your condition(s) please let us know! We would be happy to have that discussion with you and let you know if we believe a TENs Machine would be a great option for you and your condition. If you have extended health insurance and we believe a TENs should be used at home for your condition, we will help/ guide you through the entire process of getting reimbursed.
What is a TENs Machine?

How does a TENS achieve pain relief?
TRADITIONAL TENS MACHINE
Summary
Chances are very good that if you have pain or discomfort, inflammation is to blame; if not wholly, then at least in large part. Inflammation is directly involved in the pain conditions ranging from sciatica to arthritis, whiplash to tennis elbow, and from migraines to postexercise muscle soreness. So how exactly does inflammation trigger pain? Pain (like inflammation itself) is biologically and behaviorally necessary. Pain protects against tissue damage by shaping our behavior, while inflammation protects against tissue damage by mobilizing our immune system’s physiological safeguarding and repair functions. Both of these kinds of protection—behavioral and immunological—make sense when danger or damage are actually present; but both pain and inflammation can become problematic when they chronically persist after the threat is no longer around. Also, keep in mind that pain and nociception are not the same thing. Nociception is a nerve signal indicating potential mechanical, thermal, or chemical threat to tissues; pain is the experience the nervous system generates in response. Many times, when your pain is prolonged, it can be because there is continued nociceptive input (for example, from ongoing mechanical or inflammatory irritation). But pain can persist even with little or no tissue damage; or, even when there is obvious damage or degeneration, there can be little or no pain experience at all. And while your pain may not be as related to physical tissue damage as we might have thought, there turns out to be a strong relationship between pain and expectations, fears, context, memory, and social influences. These psychosocial factors play a role in inflammation, as well as in pain. For example, high hostility scores have been correlated with increased inflammation, while openness (hostility’s flip-side) has been associated with decreased inflammatory markers. But the relationship between pain, inflammation, and psychosocial factors is nuanced and complex: while depressed people often have stronger inflammatory responses, turning down inflammation with immunosuppressive drugs can also trigger depressive mood changes. So even though pain, inflammation, and reactive emotions are deeply interconnected, they don’t move in lockstep with one another. Instead, they could be thought of as different modes of physical, psychological, and behavioral adaptation that we use in varying ways in the face of perceived threat.
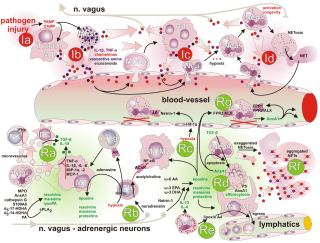
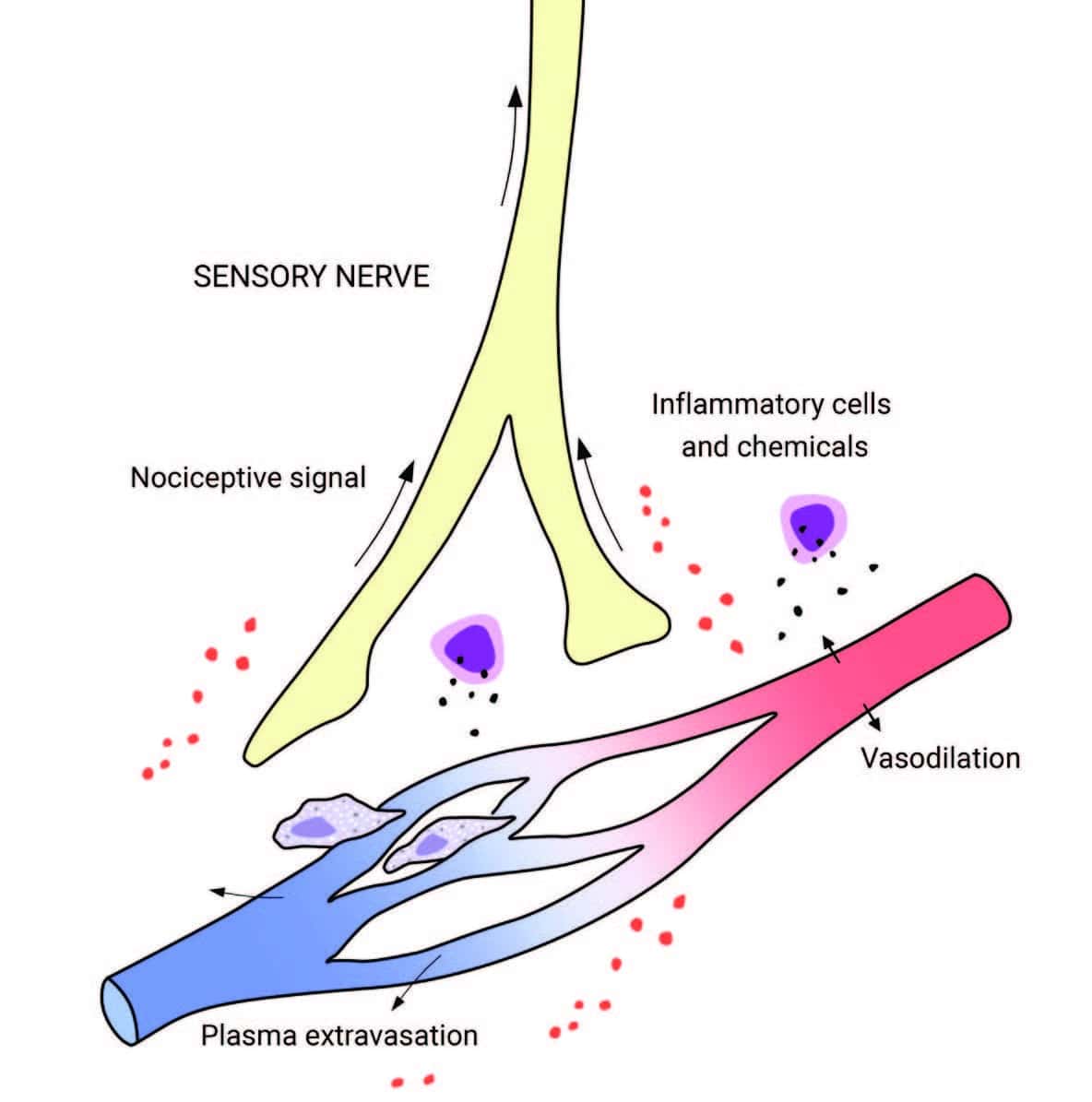
In inflammation, the nervous and immune systems work together. Tissue injury or irritation triggers the release of pro-inflammatory molecules into the surrounding interstitial environment (Image 1). Very quickly, the resulting “inflammatory soup” chemically excites and sensitizes nearby peripheral nerves, triggering behavior-altering soreness and pain (as well as initiating the cascade of the inflammatory progression, discussed in “Understanding Inflammation’s Progression”). Pain sensitization is a key link between the immune and nervous systems: sensitization amplifies the actual nociceptive signals (not just the experience of pain), either peripherally at the tissue receptors themselves, or centrally, within the spinal cord and brain. Interestingly, inflammation-related sensitization isn’t limited to the directly injured areas. Inflammatory markers have also been found within related dorsal root ganglia along the spine (associated with processing chronic pain), neural pathways within the spinal cord, and perhaps most intriguingly, even in immune cells in parts of the brain associated with both sensation and movement of the painful area (glial cells in the sensorimotor cortex, Image 2). In the short term, this neural coinflammation protectively inhibits muscular activity related to a painful (and potentially injured) area. But if prolonged, inflammatory sensitization can lead to numerous neurological and myofascial changes, including muscle size reduction (within days), reduced fatigue resistance, contraction speed change, and infiltration of fibrous and fatty tissues (within weeks or months, Image 3). Curiously, these inflammatory changes have been observed in muscles and fascia far from an injury, such as in spinal multifidus not adjacent to the site of a disk injury. In other words, inflammation doesn’t just affect the fascia and connective tissues of the locally painful area. Over time, local pain can also inflame: As bad as this sounds, the body and brain have a tremendous capacity for adaptation. This means that all these things can also get better, and hands-on work has repeatedly been shown to help. There is a lot more to say about handson work’s relevance to the inflammation/ pain relationship before we get into actual techniques. Other important concepts include the ways local pain and inflammation are affected by systemic (whole-body) inflammation, the role of the vagus nerve and autonomics, stress, the controversies around ice, diet, and much more.
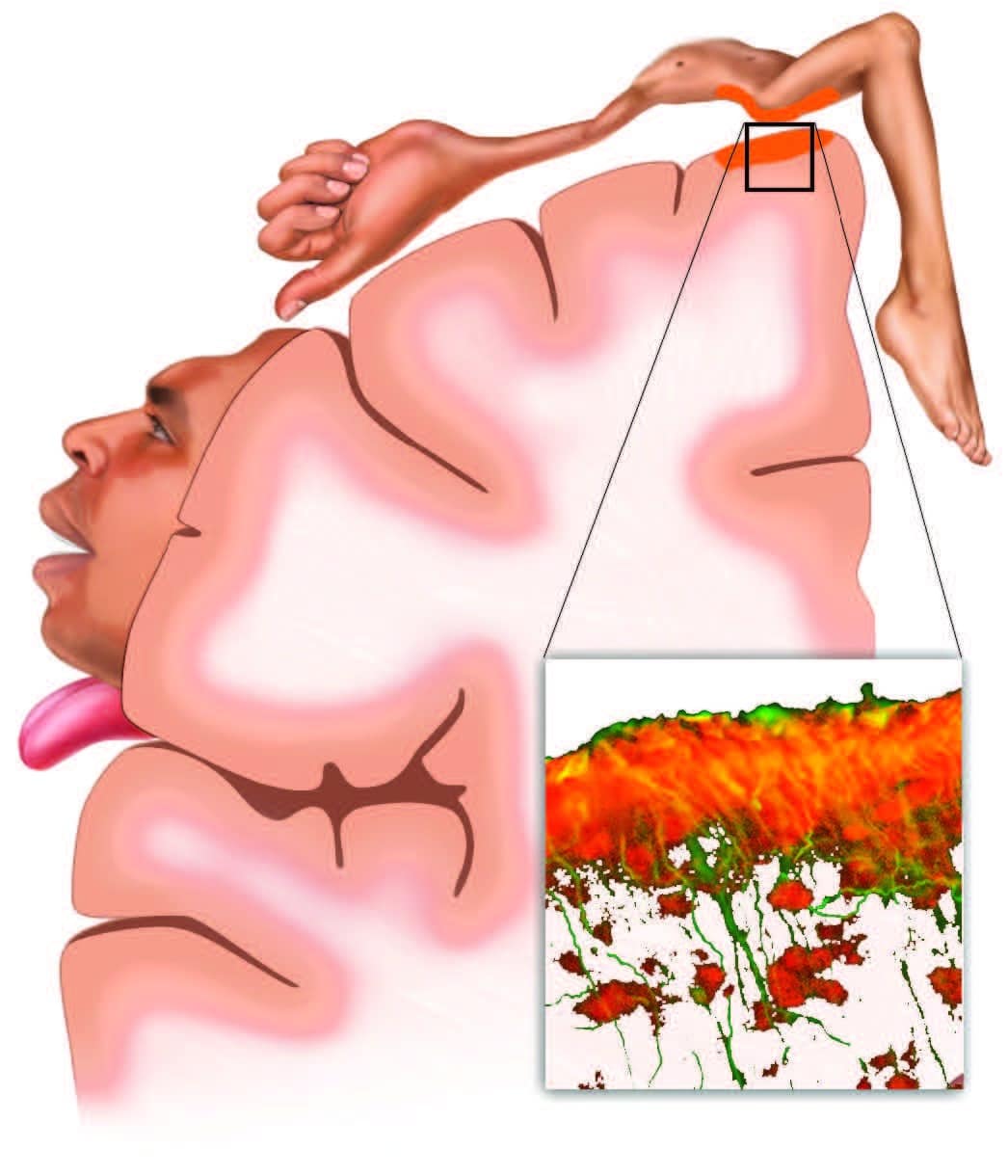
Musculoskeletal pain inflames both local tissues and the brain. In a recent study of sciatic pain (Loggia et al., 2015), inflammation wasn’t limited to the locally painful tissues of the low back and leg. Glial cells (inset), which play a key role in both immunity and chronic pain, also respond with inflammatory activation (orange) in the corresponding regions of the brain’s sensory and motor cortexes. This has implications for the use of both therapeutic sensation (such as produced by touch) and active client movement when working with musculoskeletal inflammation.
Tissue injury releases inflammatory molecules and cells into the surrounding interstitial environment. The resulting “inflammatory soup” chemically excites and sensitizes nearby sensory nerves, generating a nociceptive (pain-triggering) signal.
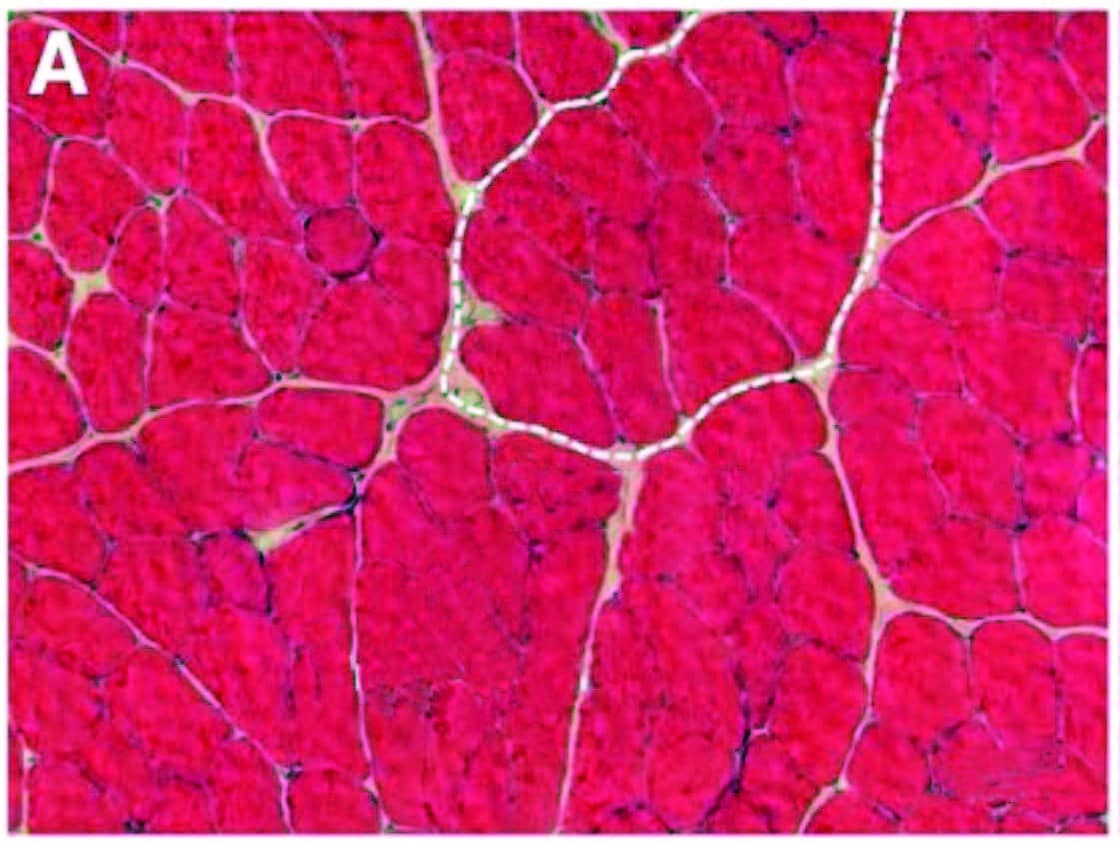
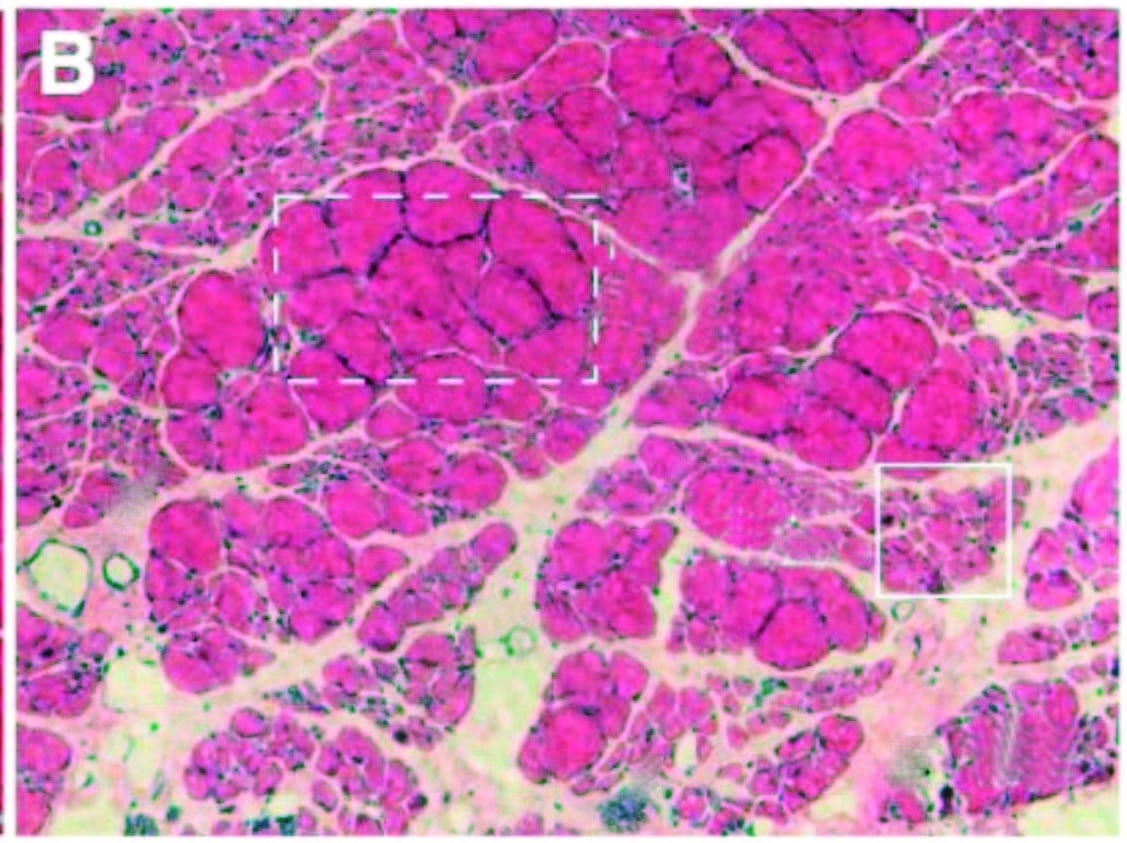
Over time, inflammation and pain can cause myofascial infiltration of fibrous and fatty tissues. A: Healthy skeletal muscle (rat tibialis anterior) with ~5 percent extracellular material. B: Fibrotic changes six months after inflammatory injury, showing extracellular increase to 20 percent of cross section. In another recent study (Bove et al., 2018), rats with repetitive strain injuries receiving modeled manual therapy (bilateral mobilization, skin rolling, and stretching) showed a reduction in nociceptor activity, neural inflammation, and fibrosis compared to unmassaged rats.
If you have any questions or concerns about your inflammation or condition, please reach out to us at 519-735-7555.
1.
2.
3.
REFERENCES
Unless you’ve really been out of the loop, you’ve surely heard inflammation is to blame for almost every known malady. Or at least, that’s what the diet, supplement, and self-help ads claim. But, even if the health-care marketplace has overhyped and oversold inflammation in its relentless search for the next big thing, it’s no exaggeration to say inflammation plays a key role in a wide range of conditions—from depression to tendon pain, fibromyalgia to cancer, and joint problems to aging itself. There are also bigger questions about the nature and function of inflammation that might influence the way we think and work. What roles do the brain and nervous system play? How do psychosocial factors (such as stress or depression) play into inflammation? Could pain itself be a type of inflammatory response? Let’s start by reviewing some inflammation basics.
Inflammation is simply the immune system’s tissue protection and repair response. It can be triggered by injury, infection, or irritation. Inflammation is necessary and good: though it can be uncomfortable, without inflammation, the body can’t protect or heal itself. Normally, inflammatory responses are self-limiting, and wind down when their protection and repair tasks are complete.
Problems happen when there is either: Chronic or unresolved inflammation is implicated in an ever-lengthening list of conditions, including asthma, Alzheimer’s, depression and mental illness, hypersensitivities, rheumatoid arthritis, metabolic syndrome, and certain cancers. Inflammation is not only a factor in nearly all chronic diseases, but also in nearly all musculoskeletal complaints, including chronic pain.
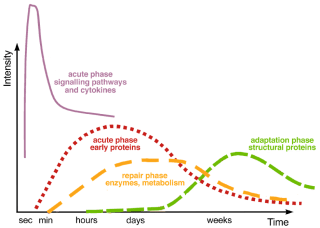
Healthy inflammatory responses progress over time, moving through acute, repair, and adaptation phases (Image 2). Though many of these functions happen simultaneously (for example, resolution processes begin even while inflammatory processes are in their early development), each step sets the stage for the next.
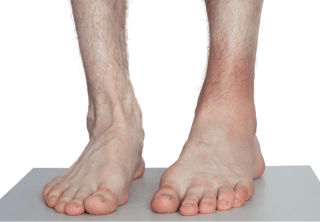
Within seconds or minutes after damage, danger, or pathogen detectors trigger an acute inflammatory response, proinflammatory histamine and kinins increase vascular and cell permeability, and the affected tissues swell with plasma and immune cells (such as leukocytes). Cytokines (signaling proteins) recruit roaming immune cells; local warmth and redness (Image 3) indicate that the active inflammatory responses are at work; and pain and tenderness (from nociceptor irritation by proinflammatories, pressure from swelling, and protective reactions) help guard against further strain or mechanical distress during this initial phase.
Within hours or days, specialized cells (such as mononuclear cells and macrophages) begin the process of degrading and removing damaged or diseased cells. Once unleashed, these destructive processes are kept in check by innate anti-inflammatory and pro-resolution mechanisms to minimize “bystander” damage to other tissues. Simultaneously, fibroblasts (which themselves have strong anti-inflammatory capacities) and other cells begin new tissue formation. Local warmth and redness diminish, as does pain—though pressure or stretch can still provoke protective tenderness as the new tissues form.
In the days, weeks, or months that follow, the proinflammatory process gradually matures and diminishes as the first-responder cells themselves become the target of degradation and cleanup via phagocytic, lymphatic, and other resolution mechanisms. Tissue strengthening continues, as fibroblasts cross-link collagen structures. Sensation normalizes and tenderness diminishes as the adapting brain gradually reevaluates and relinquishes the need for protective guarding.
Normally, inflammation progresses through acute, intermediate (repair), and later (adaptation) stages, each with its own mechanisms and processes (see text). When any stage’s progression is inhibited or prolonged, inflammation can become chronic. Resolution timelines vary tremendously, depending on the individual, the tissue, or the organ involved, etc., with full recovery in some cases taking months or years. After Steinacker et al., 1999, courtesy Advanced-Trainings.com.
Problems arise when any of these stages fails to resolve, and the inflammation becomes ongoing or chronic. This can happen when the immune system is overwhelmed by pathogens or damage, or when its resilience is taxed by stress, inactivity, diet, lack of sleep, etc. Factors such as adverse childhood experiences4 or genetics can also predispose us to chronic inflammation. But the causes of many chronic inflammatory conditions are not fully understood. These include autoimmune disorders, such as rheumatoid arthritis; fibromyalgia; and “inflamm-aging,” the low-grade inflammation-like changes that accompany aging. In prolonged inflammation:
Now that you have a better understanding of inflammation; keep an eye out NEXT WEEK for the next article “UNDERSTANDING INFLAMMATION AND PAIN” where I talk about how inflammation causes pain in both chronic and acute conditions. If you have any questions or concerns about your inflammation or condition, please reach out to us at 519-735-7555.
INFLAMMATION IS GOOD
WHEN INFLAMMATION TURNS BAD
PROGRESSION OF INFLAMMATION
ACUTE RESPONSE
REPAIR
ADAPTATION
CHRONIC INFLAMMATION