UNDERSTANDING INFLAMMATION AND PAIN
Chances are very good that if you have pain or discomfort, inflammation is to blame; if not wholly, then at least in large part.
Inflammation is directly involved in the pain conditions ranging from sciatica to arthritis, whiplash to tennis elbow, and from migraines to postexercise muscle soreness. So how exactly does inflammation trigger pain?
Pain (like inflammation itself) is biologically and behaviorally necessary. Pain protects against tissue damage by shaping our behavior, while inflammation protects against tissue damage by mobilizing our immune system’s physiological safeguarding and repair functions. Both of these kinds of protection—behavioral and immunological—make sense when danger or damage are actually present; but both pain and inflammation can become problematic when they chronically persist after the threat is no longer around.
Also, keep in mind that pain and nociception are not the same thing. Nociception is a nerve signal indicating potential mechanical, thermal, or chemical threat to tissues; pain is the experience the nervous system generates in response.
Many times, when your pain is prolonged, it can be because there is continued nociceptive input (for example, from ongoing mechanical or inflammatory irritation). But pain can persist even with little or no tissue damage; or, even when there is obvious damage or degeneration, there can be little or no pain experience at all. And while your pain may not be as related to physical tissue damage as we might have thought, there turns out to be a strong relationship between pain and expectations, fears, context, memory, and social influences.
These psychosocial factors play a role in inflammation, as well as in pain. For example, high hostility scores have been correlated with increased inflammation, while openness (hostility’s flip-side) has been associated with decreased inflammatory markers. But the relationship between pain, inflammation, and psychosocial factors is nuanced and complex: while depressed people often have stronger inflammatory responses, turning down inflammation with immunosuppressive drugs can also trigger depressive mood changes. So even though pain, inflammation, and reactive emotions are deeply interconnected, they don’t move in lockstep with one another. Instead, they could be thought of as different modes of physical, psychological, and behavioral adaptation that we use in varying ways in the face of perceived threat.
In inflammation, the nervous and immune systems work together. Tissue injury or irritation triggers the release of pro-inflammatory molecules into the surrounding interstitial environment (Image 1). Very quickly, the resulting “inflammatory soup” chemically excites and sensitizes nearby peripheral nerves, triggering behavior-altering soreness and pain (as well as initiating the cascade of the inflammatory progression, discussed in “Understanding Inflammation’s Progression”). Pain sensitization is a key link between the immune and nervous systems: sensitization amplifies the actual nociceptive signals (not just the experience of pain), either peripherally at the tissue receptors themselves, or centrally, within the spinal cord and brain.
Interestingly, inflammation-related sensitization isn’t limited to the directly injured areas. Inflammatory markers have also been found within related dorsal root ganglia along the spine (associated with processing chronic pain), neural pathways within the spinal cord, and perhaps most intriguingly, even in immune cells in parts of the brain associated with both sensation and movement of the painful area (glial cells in the sensorimotor cortex, Image 2). In the short term, this neural coinflammation protectively inhibits muscular activity related to a painful (and potentially injured) area. But if prolonged, inflammatory sensitization can lead to numerous neurological and myofascial changes, including muscle size reduction (within days), reduced fatigue resistance, contraction speed change, and infiltration of fibrous and fatty tissues (within weeks or months, Image 3).
Curiously, these inflammatory changes have been observed in muscles and fascia far from an injury, such as in spinal multifidus not adjacent to the site of a disk injury. In other words, inflammation doesn’t just affect the fascia and connective tissues of the locally painful area. Over time, local pain can also inflame:
-
- Other myofascial structures far from the injury site;
- The neurons that connect the injured areas with the brain;
- And strangely, the ankle area of your brain’s body map.
As bad as this sounds, the body and brain have a tremendous capacity for adaptation. This means that all these things can also get better, and hands-on work has repeatedly been shown to help. There is a lot more to say about handson work’s relevance to the inflammation/ pain relationship before we get into actual techniques. Other important concepts include the ways local pain and inflammation are affected by systemic (whole-body) inflammation, the role of the vagus nerve and autonomics, stress, the controversies around ice, diet, and much more.
1.
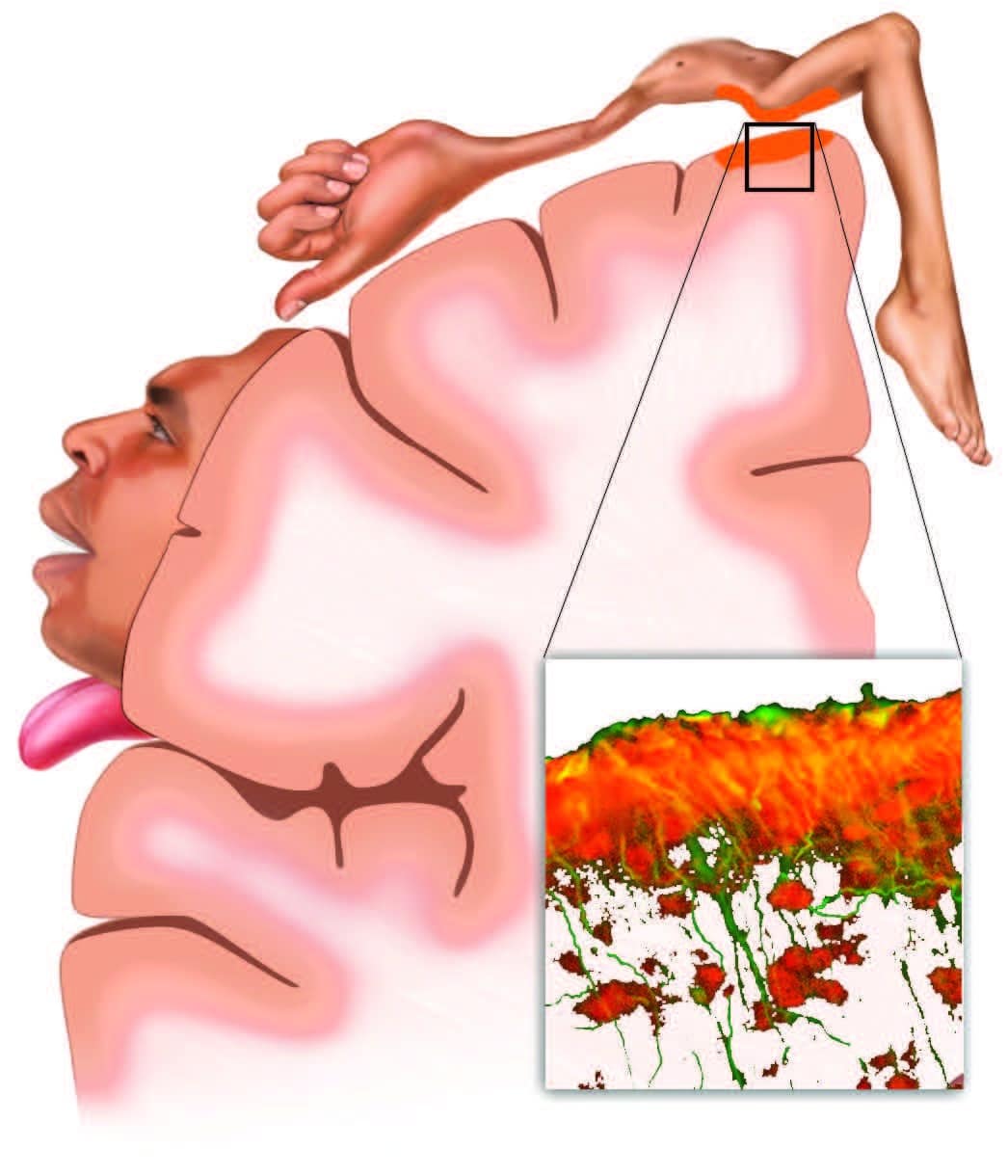
Musculoskeletal pain inflames both local tissues and the brain. In a recent study of sciatic pain (Loggia et al., 2015), inflammation wasn’t limited to the locally painful tissues of the low back and leg. Glial cells (inset), which play a key role in both immunity and chronic pain, also respond with inflammatory activation (orange) in the corresponding regions of the brain’s sensory and motor cortexes. This has implications for the use of both therapeutic sensation (such as produced by touch) and active client movement when working with musculoskeletal inflammation.
2.
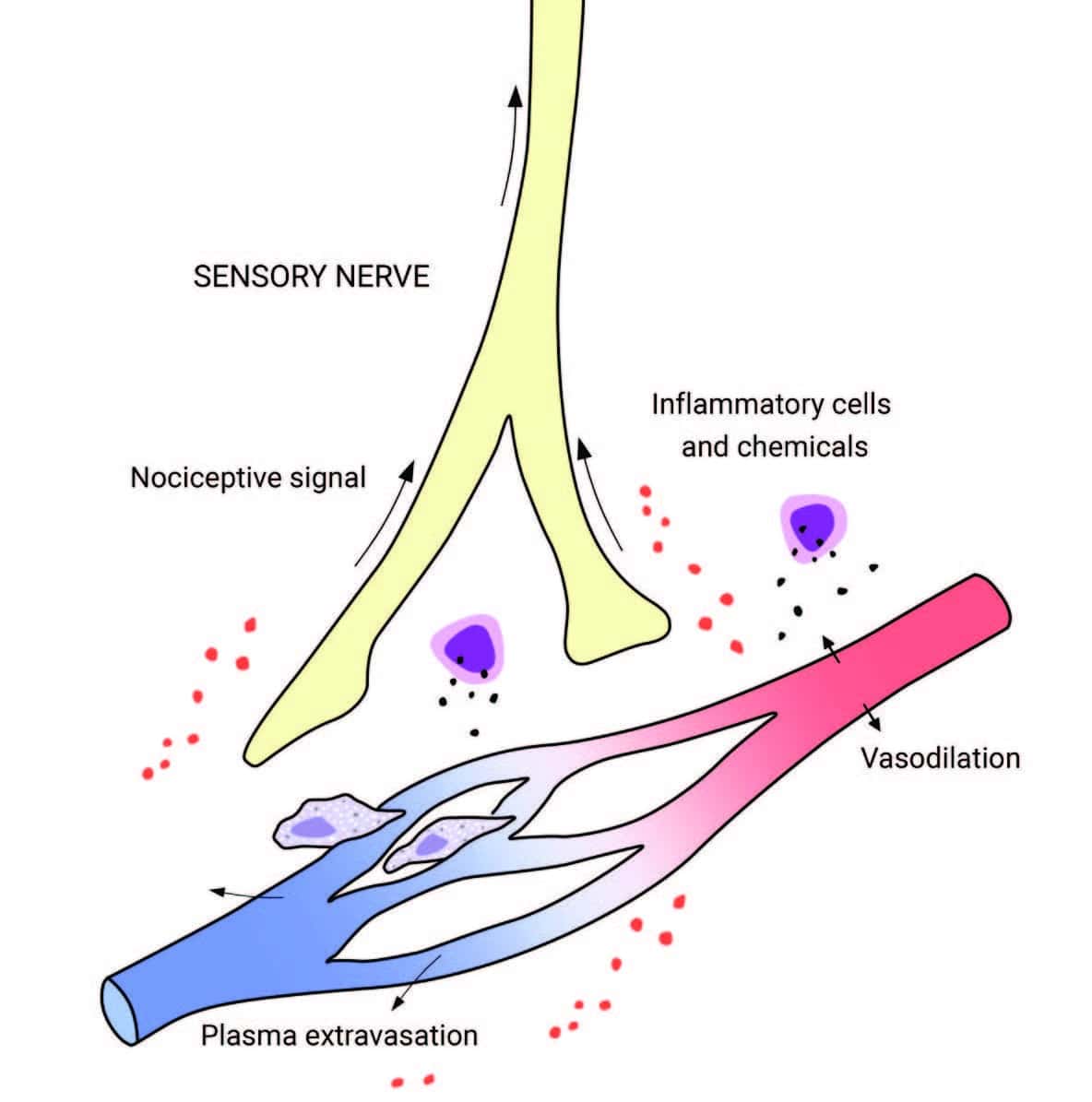
Tissue injury releases inflammatory molecules and cells into the surrounding interstitial environment. The resulting “inflammatory soup” chemically excites and sensitizes nearby sensory nerves, generating a nociceptive (pain-triggering) signal.
3.
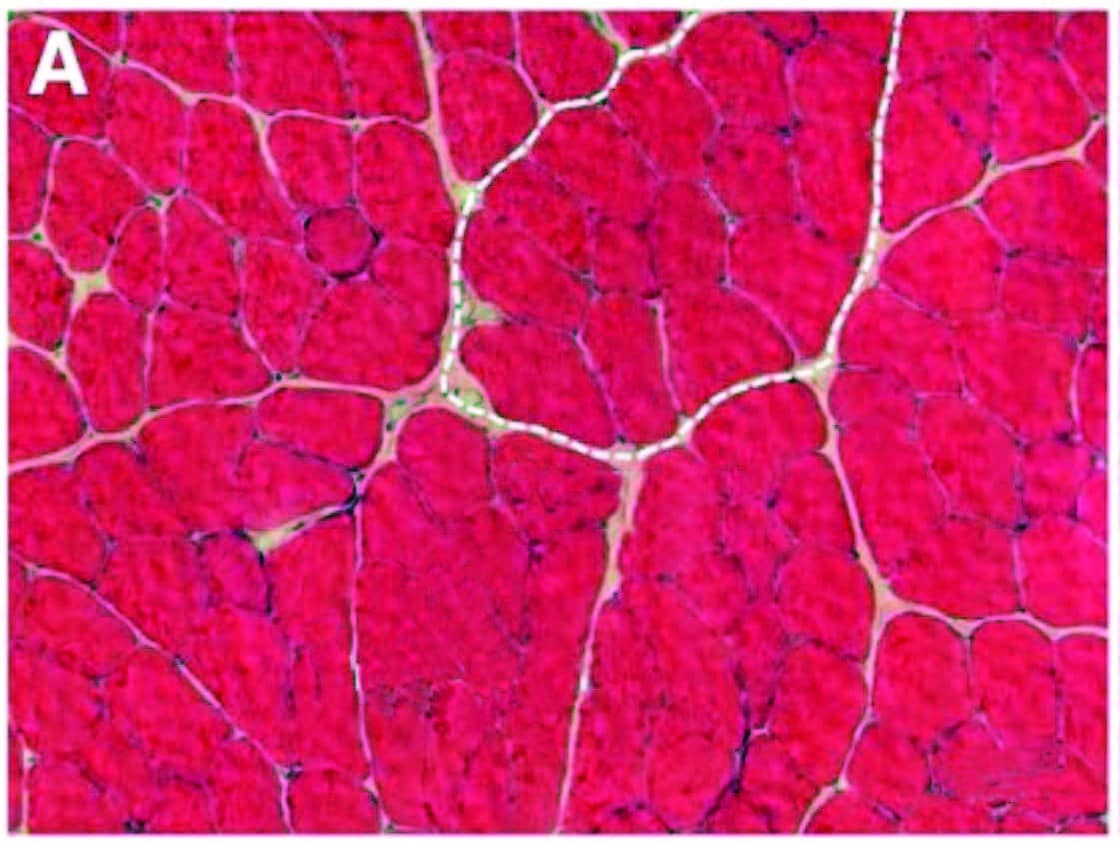
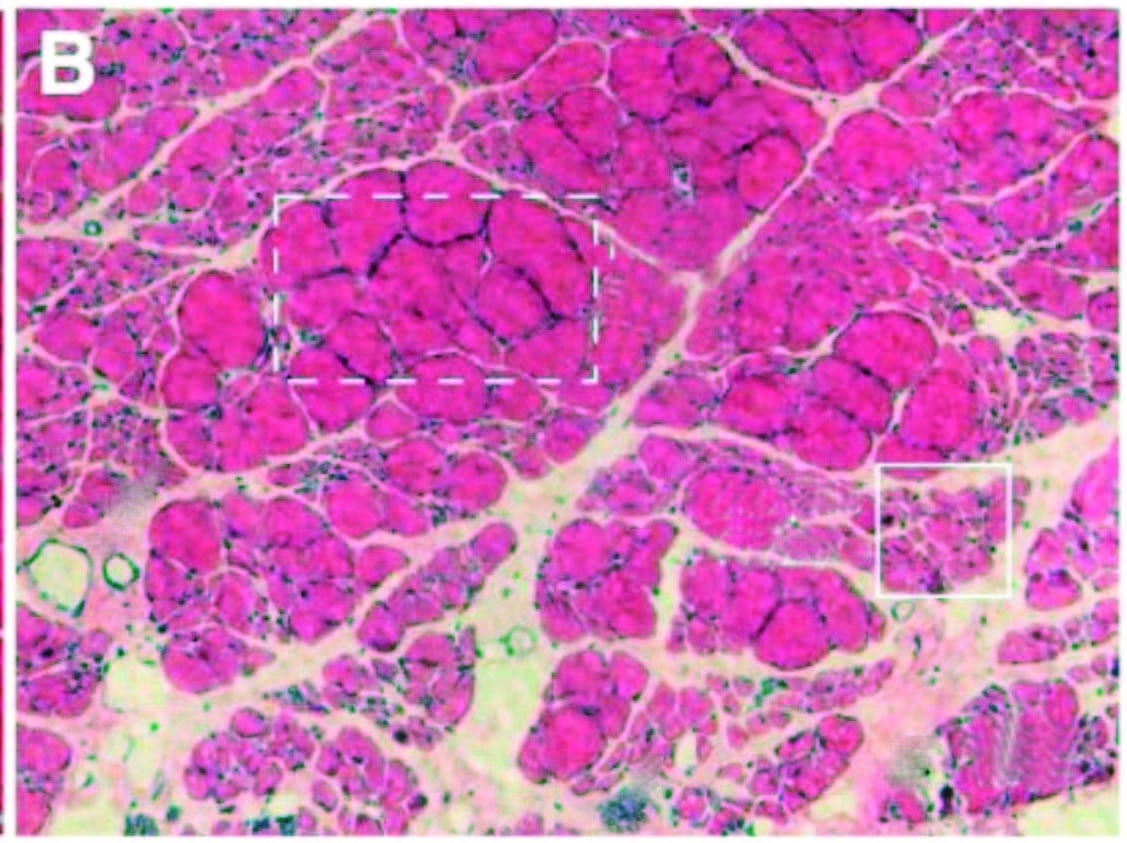
Over time, inflammation and pain can cause myofascial infiltration of fibrous and fatty tissues. A: Healthy skeletal muscle (rat tibialis anterior) with ~5 percent extracellular material. B: Fibrotic changes six months after inflammatory injury, showing extracellular increase to 20 percent of cross section. In another recent study (Bove et al., 2018), rats with repetitive strain injuries receiving modeled manual therapy (bilateral mobilization, skin rolling, and stretching) showed a reduction in nociceptor activity, neural inflammation, and fibrosis compared to unmassaged rats.
REFERENCES
- Girard et al., “Trait Hostility and Acute Infl ammatory Responses to Stress in the Laboratory,” PLoS One 11, no. 6 (2016); M. Luchetti et al., “Five-Factor Model Personality Traits and Infl ammatory Markers: New Data and a Meta-Analysis,” Psychoneuroendocrinology 50 (2014): 181–93.
- L. Loggia et al., “Evidence for Brain Glial Activation in Chronic Pain Patients,” Brain 138, no. 3 (March 2015): 604–15.
- James et al., “Dysregulation of the Infl ammatory Mediators in the Multifi dus Muscle After Spontaneous Intervertebral Disc Degeneration PARC-null Mice is Ameliorated by Physical Activity,” Spine 43, no. 20 (2018): E1,184–94. doi:10.1097/BRS.0000000000002656.
- M. Bove et al., “Manual Therapy Prevents Onset of Nociceptor Activity, Sensorimotor Dysfunction, and Neural Fibrosis Induced by a Volitional Repetitive Task, Pain (November 16, 2018): doi: 10.1097/j.pain.0000000000001443 [Epub ahead of print].
If you have any questions or concerns about your inflammation or condition, please reach out to us at
519-735-7555.


